WebTo find the amount of water (H 2 O), use the formula: ' H = (mW / T) X '. 204 0 obj
<>/Filter/FlateDecode/ID[<4976A68F0A622B4D9C11B9EE9B672A64><35BAC09DF2BBC14AA284DBAF5E8D9E97>]/Index[187 36]/Info 186 0 R/Length 82/Prev 60502/Root 188 0 R/Size 223/Type/XRef/W[1 2 1]>>stream
If you are a woman and you are pregnant, you will require more water per day and you will require even more water if you are lactating. Once we do that, we will then divide the total mass of water by the mass of the hydrate. 2. The study is critiqued for low statistical power and biases due to partial unblinding of participants by Price A. and Burls A. m = number of moles of water. Such measures of expenditure use highly accurate methods and thus TDEE has been set based on solid data rather than the compromise inherent in the Adequate Intake estimations made for water. At 1,000 psia, the hydrate formation temperature is 61F at a gas gravity of 0.603. If you eat a lot of these foods each day, you wont need to drink quite as much. 187 0 obj
<>
endobj
In the example, notice that you should be drinking more than 12 glasses of water, not eight! http://dx.doi.org/10.1016/S1472-7862(03)00063-7, http://dx.doi.org/10.1016/S0378-3812(01)00520-9, https://webevents.spe.org/products/flow-assurance-managing-flow-dynamics-and-production-chemistry-2, https://petrowiki.spe.org/w/index.php?title=Predicting_hydrate_formation&oldid=53681, 3.4.1 Inhibition and remediation of hydrates, scale, paraffin or wax, and asphaltene, Copyright 2012-2023, Society of Petroleum Engineers, the enthalpy of water in the hydrocarbon solution minus that of pure liquid water, Btu/lbm, enthalpy difference across a valve or restriction, Btu/lbm, amount of inhibitor in the vapor or liquid hydrocarbon phases, a Katzs value term, defined as a components mole fraction divided by that in the hydrate, DePriesters vapor/liquid value, defined as a components mole fraction divided by that in the liquid, the average molecular weight of a gas in a mixture, in the Gibbs phase rule, the number of phases in a nonreacting system, wt% of the inhibitor in the free-water phase, mole fraction for hydrocarbon in liquid water, mole fraction MeOH in the free-water phase, mole fraction for water in liquid hydrocarbon, hydrate temperature depression below the equilibrium temperature at a given pressure, F, Those which enable the prediction of the pressure and temperature at which hydrates begin to form (incipient hydrate formation programs), Those which predict all phases and amounts at higher pressures and lower temperatures than the incipient hydrate formation point (flash programs, or Gibbs energy minimization programs). WebThe hydrate's molecular weight is 381.365 g/mol The total amount of water in the molecular weight is 180.148 g. 180.148 / 381.365 = 0.47238 (decimal amount of the hydrate that is water) (5.00 g) (0.47238) = 2.3619 g (this is the water lost) 5.00 g 2.3619 g = 2.6381 g of the anhydrous Na2B4O7remaining Round off to three sig figs: 2.64 g Water intake acutely reduces heart rate and increases blood pressure in people with normal or increased blood pressure. Epsom salts is M g S O X 4 x H X 2 O. 7 Permissible expansion of a 0.8-gravity natural gas without hydrate formation (from Katz[9]). [11] Vij V.A.K., Joshi A.S. (2014) "Effect of excessive water intake on body weight, body mass index, body fat, and appetite of overweight female participants" Journal of Natural Science, Biology, and Medicine 5(2):340344. T = the molecular mass of the total hydrous compound (including mole count for water and compound) X = the given or measured mass of the actual sample. About 11.5 cups (2.7 liters) of fluids a day for women. The temperature at which hydrates form at 6.8 MPa (1,000 psia). qxnx@jxdJJQw? Divide the number of moles of water lost by the number of moles of anhydrous salt to get the ratio of water molecules to formula units. WebHydration Calculator - Mindful by Sodexo Your body depends on water for survival. The formula for our hydrate is FeCl 3 6H 2 O. However, research on this hypothesis is limited and merits further exploration. Fig. 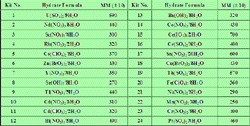 Our bodies constantly lose water as part of the metabolic processes. Methanol will exist in three phases: Step 1Calculate hydrate formation conditions using the gas gravity chart. Thus, water drinking induced increase in sympathetic activity is an important and unrecognized component of daily energy expenditure and hence weight loss. Fig. 3) and the gas enthalpy/entropy charts by Brown[12] to determine Fig. With that inhibitor concentration as a basis, the amount of inhibitor in the vapor or liquid hydrocarbon phases is estimated by: With Eq.
Our bodies constantly lose water as part of the metabolic processes. Methanol will exist in three phases: Step 1Calculate hydrate formation conditions using the gas gravity chart. Thus, water drinking induced increase in sympathetic activity is an important and unrecognized component of daily energy expenditure and hence weight loss. Fig. 3) and the gas enthalpy/entropy charts by Brown[12] to determine Fig. With that inhibitor concentration as a basis, the amount of inhibitor in the vapor or liquid hydrocarbon phases is estimated by: With Eq.  In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). 5 Permissible expansion of a 0.6-gravity natural gas without hydrate formation (from Katz[9]). Figs. Therefore we need to frequently replenish the fluids in our system for it to function optimally (see the benefits of optimal hydration). WebFree Chemistry calculator - Calculate chemical reactions and chemical properties step-by-step Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. Find Out How Much Water You Need to Drink per Day To give yourself a better sense of how much water you need to drink each day, use this hydration calculator. https://webevents.spe.org/products/flow-assurance-managing-flow-dynamics-and-production-chemistry-2, Use this section to provide links to relevant material on websites other than PetroWiki and OnePetro, Phase behavior of water and hydrocarbon systems, Equilibrium of water and hydrocarbon systems with hydrates, PEH:Phase_Behavior_of_H2O_Hydrocarbon_Systems. WebWhat is the formula of the hydrate? 5 through 7 occur at the upstream pressure of 40.8 MPa (6,000 psia), the Joule-Thomson inversion pressure. For the two-phase regions of hydrate equilibria (i.e., V-H, LHC-H, and I-H), the key question is that of water content: how much water can a vapor or liquid hydrocarbon phase hold before hydrates will precipitate? A gas is composed of the following (in mole percent): When free water is present with the gas, find: Solution. Calculate the value of x. Zinc nitrate Z n ( N O X 3) X 2 x H X 2 O contains 21.98 % zinc by mass. These are population-wide adequate intake estimations and are thus less preferred than the personalized calculations from our water intake calculator above. 3.52 g 1 moleBaCl2 208.2 g = 0.017 moles The mole ratio between the water and the anhydrous salt is moles of water moles of anhydrate = 0.034 0.017 = 2 This means that for every mole of BaCl2, you have 2 moles of water. m = number of moles of water. Thats because hydration needs are far from one-size-fits-all. The gas flowing through the pipeline is cooled to 38F by the surrounding water. We will then add the mass of the water to the mass of anhydrous salt to get the total mass of the hydrate. xyUt,6lfN)N-m-"Y
W = the mass of water in one mole of the compound. The pressure at which hydrates will form then is read directly from the chart at that gas gravity and temperature. al [9] suggests that considering the observed positive subjective effects, it seems reasonable to recommend headache patients to try this non-invasive intervention for a short period of time to see whether they experience improvement. Use This Chart for Water Content of Natural Gases. In Fig. 22.4 cm3 of the acid was required. 6). If you are asking yourself how much water should you drink per day our water calculator will calculate that for you in cups (glasses), ounces (oz), and milliliters. 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Four-phase (LW-H-V-LHC) hand calculation methods are not available, and one generally must rely on computer methods for this most common flow assurance hydrate concern. endstream
endobj
191 0 obj
<>stream
Is Your Symptom a Sign of Dehydration or Something Else? The Next Generation of Hydrate Prediction: An Overview. O. Whether you need help solving quadratic equations, inspiration for the upcoming science fair or the latest update on a major storm, Sciencing is here to help. [12] Daniels M. C., Popkin B. M. (2010) "The impact of water intake on energy intake and weight status: a systematic review" Nutrition Reviews 68(9):505521. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. where: H = the mass of water in the sample. New York: Chemical Engineering Progress Symposium Series, American Institute of Chemical Engineers. 3. What molecular formula represents a carbohydrate?
In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). 5 Permissible expansion of a 0.6-gravity natural gas without hydrate formation (from Katz[9]). Figs. Therefore we need to frequently replenish the fluids in our system for it to function optimally (see the benefits of optimal hydration). WebFree Chemistry calculator - Calculate chemical reactions and chemical properties step-by-step Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. Find Out How Much Water You Need to Drink per Day To give yourself a better sense of how much water you need to drink each day, use this hydration calculator. https://webevents.spe.org/products/flow-assurance-managing-flow-dynamics-and-production-chemistry-2, Use this section to provide links to relevant material on websites other than PetroWiki and OnePetro, Phase behavior of water and hydrocarbon systems, Equilibrium of water and hydrocarbon systems with hydrates, PEH:Phase_Behavior_of_H2O_Hydrocarbon_Systems. WebWhat is the formula of the hydrate? 5 through 7 occur at the upstream pressure of 40.8 MPa (6,000 psia), the Joule-Thomson inversion pressure. For the two-phase regions of hydrate equilibria (i.e., V-H, LHC-H, and I-H), the key question is that of water content: how much water can a vapor or liquid hydrocarbon phase hold before hydrates will precipitate? A gas is composed of the following (in mole percent): When free water is present with the gas, find: Solution. Calculate the value of x. Zinc nitrate Z n ( N O X 3) X 2 x H X 2 O contains 21.98 % zinc by mass. These are population-wide adequate intake estimations and are thus less preferred than the personalized calculations from our water intake calculator above. 3.52 g 1 moleBaCl2 208.2 g = 0.017 moles The mole ratio between the water and the anhydrous salt is moles of water moles of anhydrate = 0.034 0.017 = 2 This means that for every mole of BaCl2, you have 2 moles of water. m = number of moles of water. Thats because hydration needs are far from one-size-fits-all. The gas flowing through the pipeline is cooled to 38F by the surrounding water. We will then add the mass of the water to the mass of anhydrous salt to get the total mass of the hydrate. xyUt,6lfN)N-m-"Y
W = the mass of water in one mole of the compound. The pressure at which hydrates will form then is read directly from the chart at that gas gravity and temperature. al [9] suggests that considering the observed positive subjective effects, it seems reasonable to recommend headache patients to try this non-invasive intervention for a short period of time to see whether they experience improvement. Use This Chart for Water Content of Natural Gases. In Fig. 22.4 cm3 of the acid was required. 6). If you are asking yourself how much water should you drink per day our water calculator will calculate that for you in cups (glasses), ounces (oz), and milliliters. 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Four-phase (LW-H-V-LHC) hand calculation methods are not available, and one generally must rely on computer methods for this most common flow assurance hydrate concern. endstream
endobj
191 0 obj
<>stream
Is Your Symptom a Sign of Dehydration or Something Else? The Next Generation of Hydrate Prediction: An Overview. O. Whether you need help solving quadratic equations, inspiration for the upcoming science fair or the latest update on a major storm, Sciencing is here to help. [12] Daniels M. C., Popkin B. M. (2010) "The impact of water intake on energy intake and weight status: a systematic review" Nutrition Reviews 68(9):505521. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. where: H = the mass of water in the sample. New York: Chemical Engineering Progress Symposium Series, American Institute of Chemical Engineers. 3. What molecular formula represents a carbohydrate? 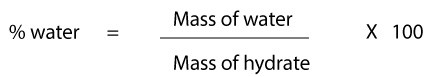 5) Mass of hydrated salt mass of anhydrous salt = mass of water. Let's look at our data: Let's look at our data: Mass of the empty dish used for weighing = 2.5 g In this lab we actually calculate the formula of the formula for the hydrate MgSO 4 x H 2 O The x is how many waters are attached to each MgSO 4. Drinking water gradually throughout the day is important. The kidneys function more efficiently in the presence of an abundant water supply. You take a sample of the hydrate, weigh it, and find it has a mass of 4.13 g. You then heat the sample in order to remove the water and find the weight of the anhydrate to be 3.52 g. The first thing to do is to determine the moles of water that were in the sample, #(4.13-3.52)# #"g" * ("1 mole water")/("18.0 g") = 0.034# #"moles"#, You now go on to determine the moles of the anhydrous salt, #BaCl_2#, #3.52# #"g" * ("1 mole"BaCl_2)/("208.2 g") = 0.017# #"moles"#, The mole ratio between the water and the anhydrous salt is, #("moles of water")/("moles of anhydrate") = 0.034/0.017=2#. [7] It would therefore seem that getting close to your optimal hydration level with the help of a hydration calculator can have benefits for your mental power. The difference between the original water and the water remaining in the gas is the mass of liquid water from condensation: 600 9 = 591 lbm/MMscf.
5) Mass of hydrated salt mass of anhydrous salt = mass of water. Let's look at our data: Let's look at our data: Mass of the empty dish used for weighing = 2.5 g In this lab we actually calculate the formula of the formula for the hydrate MgSO 4 x H 2 O The x is how many waters are attached to each MgSO 4. Drinking water gradually throughout the day is important. The kidneys function more efficiently in the presence of an abundant water supply. You take a sample of the hydrate, weigh it, and find it has a mass of 4.13 g. You then heat the sample in order to remove the water and find the weight of the anhydrate to be 3.52 g. The first thing to do is to determine the moles of water that were in the sample, #(4.13-3.52)# #"g" * ("1 mole water")/("18.0 g") = 0.034# #"moles"#, You now go on to determine the moles of the anhydrous salt, #BaCl_2#, #3.52# #"g" * ("1 mole"BaCl_2)/("208.2 g") = 0.017# #"moles"#, The mole ratio between the water and the anhydrous salt is, #("moles of water")/("moles of anhydrate") = 0.034/0.017=2#. [7] It would therefore seem that getting close to your optimal hydration level with the help of a hydration calculator can have benefits for your mental power. The difference between the original water and the water remaining in the gas is the mass of liquid water from condensation: 600 9 = 591 lbm/MMscf.  Cold climates have less of an effect, but extremely cold climates may result in increased energy needs to compensate for the heat loss and thus you may need a higher water intake per day. WebCamelBak has your hydration needs covered from kids water bottles and sports water bottles to travel mugs and drinkware for your car, home or classroom. Inadequate fluid consumption is touted as a common culprit in constipation and increasing fluid intake is a frequently recommended treatment. 6UBY]e2LeKBACM!SONslN?FkjTKqG6X.5s'+[h1W{Hen RM2S,J
y:raJizFr>#[| OL^u~$QAH{B!Lvw2y6An3(O$_SdE|mqjqTMiLHdbwCb ib=C#L`FJz f;Fv8}#[m%vsm +TXiNQ?3K*U~0 u
In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). 21 ff ) contains a more accurate computer calculation method and discussion. Hydrate formation with rapid expansion from a wet line is common in fuel gas or instrument gas lines. HTn0+Hh$-PKSVM,-Y@rwv8;P9#FccY?b|s]^~{IaqlpJjQ5-MTs5}NN9N[\0m1
MX4I)h$l:a #QHn9w=G@ -calculate the mass of the water that has been removed; |lx,dDCQ~C? As theAmerican College of Sports Medicinepoints out, the intensity and duration of exercise affects how much you sweat and your subsequent fluid needs. WebThe hydrate's molecular weight is 381.365 g/mol The total amount of water in the molecular weight is 180.148 g. 180.148 / 381.365 = 0.47238 (decimal amount of the hydrate that is water) (5.00 g) (0.47238) = 2.3619 g (this is the water lost) 5.00 g 2.3619 g = 2.6381 g of the anhydrous Na2B4O7remaining Round off to three sig figs: 2.64 g The gas also experiences a pressure drop to 950 psia. pc0&xBf)ZB'p04F$dl:,vI!llYn{8Kj.,ouiBbw/2LZu5\D\jGdrlluSw( nXW5VE}khy:b]&brwm%_v]Nu4uE]^lVuIh2P/,|j5mb$V s3/I|@Iie{5p!9BbDB2!~=&PCgf9xYjY1v,mM.#HfH$ex+77| These programs are of two types: Of these two program types, the flash/Gibbs type is gaining pre-eminence because its predictions are available in the phase diagram interior (where many systems operate), whereas the incipient type provides the pressure/temperature (P/T) points of hydrate initiation. Two rapid expansion curves for the same 0.6-gravity gas are shown in Fig. Fig. Whole, nutrient-rich foods and beverages, including the following, also count toward your fluid intake: On the other hand, alcohol is dehydrating and does not count as fluid. Hypohydration appears to have a more significant impact on high-intensity and endurance activity such as tennis and long-distance running than on anaerobic activities such as weight lifting or on shorter-duration activities, such as rowing [1]. The result is constant enthalpy (H2 = 0) operation on expansion. 0 By ShapeFit Fitness Calculators This hydration calculator provides an estimate for the amount of water you should consume when exercising in order to stay fully hydrated and avoid becoming dehydrated during your workout. One mole of carbonate ion will produce n moles of water. Living in a climate which is too hot or too cold, or being pregnant are also important factors. The calculator will work out your hydration level based on the information you give about yourself and your daily drinking habits. Find out if your water intake is adequate by using this simple tool. The hydrate was found to contain 71.4 % oxygen. Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Two moles of HCl react for every one mole of carbonate. Enter the chemical formula of a compound to calculate its molar mass and elemental composition. Most of the studies are observational and there is a distinct lack of randomized controlled trials and long-term studies of the weight loss effects of replacing other beverages with water. We are also providing tools that will help the process and transition to building habits of drinking more natural water with infusers that make water taste more like flavours you are familiar with. For example, the chemical formula of anhydrous copper (II) sulfate is Cu(SO4). WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. Symptoms, Causes, Diagnosis, Treatment, and Prevention. A clear, prescriptive method for constructing the hydrate flash program has recently been published. See all questions in Empirical and Molecular Formulas. If you are asking yourself how much water should you drink per day our water calculator will calculate that for you in cups (glasses), ounces (oz), and milliliters. Water comprises from 75% body weight in infants to 55% in elderly and is essential for cellular homeostasis and life so it is no wonder water is so important to one's health. The molar mass of this compound is equal to the molar mass of copper plus the molar mass of sulfur plus four times the molar mass of oxygen (since there are four oxygen atoms in the molecule). Which hydrate conditions are calculable by hand? HUMo0Wh
3k@kn[ZHv7
v-SD>5W8Dh`2 Since water and beverages are only a part of the input, our calculator will output both your total water intake recommendation as well as how much of it you need to get through drinking fluids.
Cold climates have less of an effect, but extremely cold climates may result in increased energy needs to compensate for the heat loss and thus you may need a higher water intake per day. WebCamelBak has your hydration needs covered from kids water bottles and sports water bottles to travel mugs and drinkware for your car, home or classroom. Inadequate fluid consumption is touted as a common culprit in constipation and increasing fluid intake is a frequently recommended treatment. 6UBY]e2LeKBACM!SONslN?FkjTKqG6X.5s'+[h1W{Hen RM2S,J
y:raJizFr>#[| OL^u~$QAH{B!Lvw2y6An3(O$_SdE|mqjqTMiLHdbwCb ib=C#L`FJz f;Fv8}#[m%vsm +TXiNQ?3K*U~0 u
In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). 21 ff ) contains a more accurate computer calculation method and discussion. Hydrate formation with rapid expansion from a wet line is common in fuel gas or instrument gas lines. HTn0+Hh$-PKSVM,-Y@rwv8;P9#FccY?b|s]^~{IaqlpJjQ5-MTs5}NN9N[\0m1
MX4I)h$l:a #QHn9w=G@ -calculate the mass of the water that has been removed; |lx,dDCQ~C? As theAmerican College of Sports Medicinepoints out, the intensity and duration of exercise affects how much you sweat and your subsequent fluid needs. WebThe hydrate's molecular weight is 381.365 g/mol The total amount of water in the molecular weight is 180.148 g. 180.148 / 381.365 = 0.47238 (decimal amount of the hydrate that is water) (5.00 g) (0.47238) = 2.3619 g (this is the water lost) 5.00 g 2.3619 g = 2.6381 g of the anhydrous Na2B4O7remaining Round off to three sig figs: 2.64 g The gas also experiences a pressure drop to 950 psia. pc0&xBf)ZB'p04F$dl:,vI!llYn{8Kj.,ouiBbw/2LZu5\D\jGdrlluSw( nXW5VE}khy:b]&brwm%_v]Nu4uE]^lVuIh2P/,|j5mb$V s3/I|@Iie{5p!9BbDB2!~=&PCgf9xYjY1v,mM.#HfH$ex+77| These programs are of two types: Of these two program types, the flash/Gibbs type is gaining pre-eminence because its predictions are available in the phase diagram interior (where many systems operate), whereas the incipient type provides the pressure/temperature (P/T) points of hydrate initiation. Two rapid expansion curves for the same 0.6-gravity gas are shown in Fig. Fig. Whole, nutrient-rich foods and beverages, including the following, also count toward your fluid intake: On the other hand, alcohol is dehydrating and does not count as fluid. Hypohydration appears to have a more significant impact on high-intensity and endurance activity such as tennis and long-distance running than on anaerobic activities such as weight lifting or on shorter-duration activities, such as rowing [1]. The result is constant enthalpy (H2 = 0) operation on expansion. 0 By ShapeFit Fitness Calculators This hydration calculator provides an estimate for the amount of water you should consume when exercising in order to stay fully hydrated and avoid becoming dehydrated during your workout. One mole of carbonate ion will produce n moles of water. Living in a climate which is too hot or too cold, or being pregnant are also important factors. The calculator will work out your hydration level based on the information you give about yourself and your daily drinking habits. Find out if your water intake is adequate by using this simple tool. The hydrate was found to contain 71.4 % oxygen. Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Two moles of HCl react for every one mole of carbonate. Enter the chemical formula of a compound to calculate its molar mass and elemental composition. Most of the studies are observational and there is a distinct lack of randomized controlled trials and long-term studies of the weight loss effects of replacing other beverages with water. We are also providing tools that will help the process and transition to building habits of drinking more natural water with infusers that make water taste more like flavours you are familiar with. For example, the chemical formula of anhydrous copper (II) sulfate is Cu(SO4). WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. Symptoms, Causes, Diagnosis, Treatment, and Prevention. A clear, prescriptive method for constructing the hydrate flash program has recently been published. See all questions in Empirical and Molecular Formulas. If you are asking yourself how much water should you drink per day our water calculator will calculate that for you in cups (glasses), ounces (oz), and milliliters. Water comprises from 75% body weight in infants to 55% in elderly and is essential for cellular homeostasis and life so it is no wonder water is so important to one's health. The molar mass of this compound is equal to the molar mass of copper plus the molar mass of sulfur plus four times the molar mass of oxygen (since there are four oxygen atoms in the molecule). Which hydrate conditions are calculable by hand? HUMo0Wh
3k@kn[ZHv7
v-SD>5W8Dh`2 Since water and beverages are only a part of the input, our calculator will output both your total water intake recommendation as well as how much of it you need to get through drinking fluids.  Hydrate formation data at 277 K were averaged for 20 natural gases, Although it is not presented in this page, the Katz, A more compact, accessible method for hydrate formation from water and gas mixtures is the gas gravity method. At 50F, the hydrate formation pressure is 450 psia at a gas gravity of 0.603. Unfortunately, calculating exact hydration losses from physical activity is complicated, because people sweat at drastically different rates, according to theAmerican College of Sports Medicine. Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope [1]. Solving this equation yields m =228.7 lbm MeOH in the water phase. Water is ejected from the body in the form of urine, as result of gastrointestinal processes, as part of respiration, as well as through sweating and other insensible outputs. Step 3Calculate the mass of liquid water/MMscf of natural gas. f$)},)22ynCxxb1 >5/jS$C9EGzyQT [D|V,j_{J@pt:*_VuZjBJb1Z;"aD\MCHO>y^zzw]dO}AuN{7m(-u }CDBPMS 3, calculate the gas gravity and specify the lowest temperature of the pipeline/process. WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. For example, at a typical seafloor temperature of 277 K, hydrates will form in a natural gas system if free water is available and the pressure is greater than 1.2 MPa. Similarly, when someone is pregnant, they require additional fluids to maintain amniotic fluid levels and keep the baby growing steadily, asPennStatediscusses. If you are thirsty, you should certainly drink water no matter what any kind of calculation or chart is telling you about how much water per day you need to drink. %%EOF
Why Improve Hydrate Predictions for Deepwater Black Oil? Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. Note that maxima in Figs. While water intake needs vary, one thing is for sure: Meeting your personal hydration needs each day will have a tremendous benefit to your health. The basis for both program types is a hydrate equation of state (EOS). WebUse this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. Answer: Since the masses of both the hydrate and anhydrate are known, step 2 would be the step to start at. Did you know that water makes up more than half of your body weight? litres per day). Produced free water enters the pipeline at a rate of 0.25 B/D. Caution: this method is only approximate for several reasons: Calculate the number of water molecules associated with each formula unit of cobalt (II) nitrate. Hydrates are compounds that contain water with a definite mass in the form of #H_2O# in their molecular formula. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 WebCamelBak has your hydration needs covered from kids water bottles and sports water bottles to travel mugs and drinkware for your car, home or classroom. Decrements in physical performance in athletes have been observed under much lower levels of dehydration: as little as 2% Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort. 2. Hydrates are compounds that contain water with a definite mass in the form of H_2O in their molecular formula. WebHydration Calculator - Mindful by Sodexo Your body depends on water for survival. 3) provides the MeOH or MEG concentration in the aqueous phase. The most accurate predictions of hydrate formation conditions are made using commercial phase equilibria computer programs. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. Every cell, tissue and organ in your body needs water to function correctly. 1959. In our example, 16 grams / 160 grams per mole = 0.1 moles. Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. The Enter the chemical formula of a compound to calculate its molar mass and elemental composition. For example, your body uses water to maintain its temperature, remove waste and lubricate joints. Clathrate Hydrates of Natural Gases, second edition. Every cell, tissue and organ in your body needs water to function correctly. 160, SPE-945140-G, 140. The calculator will work out your hydration level based on the information you give about yourself and your daily drinking habits. hbbd``b`Z
$[A-`m / 1 B-CH.(d100R 3.52 g 1 moleBaCl2 208.2 g = 0.017 moles The mole ratio between the water and the anhydrous salt is moles of water moles of anhydrate = 0.034 0.017 = 2 This means that for every mole of BaCl2, you have 2 moles of water. 2: Using this gas gravity number to read Fig. Unit system (2015) "Water intake: validity of population assessment and recommendations" European Journal of Nutrition, 54(Suppl 2):1116, [4] Rehrer N.J., Burke L.M. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 V*+ou)a1U j!UiHeTrI,h\OUM!sOb p\C>.G`Hv4i4!J Epsom salts is M g S O X 4 x H X 2 O. 11.9 to be 38F (497.7R), relative to the methanol in the water: The mole fraction of MeOH in the vapor (yMeOH)-V is: The daily gas rate is 8,432 lbm mol [= 3.2 106 scf/(379.5 scf/lbm mol), where an scf is at 14.7 psia and 60F], so that the MeOH lost to the gas is 4.29 lbm mol (= 0.000509 8,432) or 137.3 lbm/D. The basis for these calculations is 1.0 MMscf/D. These compounds often come in the form of a crystal which can then be heated in order to remove the water in the form of steam. Use this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. How do u find the percent error? For example, your body uses water to maintain its temperature, remove waste and lubricate joints. WebHydration Calculator Follow 3 easy steps to see whether you are drinking enough water. HTn0+HKREA%9(HYR/>h(k8vR|+JE+j,5+duUUn%(mZqCh;lR0%UmcRk01bKUyg$>VZ{>|Zil
2J_u,Q'!hh,J>/r-NesrQFk*2UJv6V OqYFdH$U^7Rjl"~6UjMx:}'K
|>dM|,y=Ho %tJMD\=B=B#J'\E]"Haxu j;=B{Edql&05X4-E;2j3nb9no=G;pe>QYO=u =9q kS|E In the example, notice that you should be drinking more than 12 glasses of water, not eight! Based in San Diego, John Brennan has been writing about science and the environment since 2006. Each tool is carefully developed and rigorously tested, and our content is well-sourced, but despite our best effort it is possible they contain errors. Energy requirements are strongly evidence-based in each age and gender group based on extensive research which takes into account body size and activity level which are crucial determinants of energy expenditure which must be met by dietary energy intake. 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Definite mass in the aqueous phase program has recently been published gas gravity of 0.603, 16 /. % % EOF Why Improve hydrate Predictions for Deepwater Black Oil yields m =228.7 lbm MeOH the. For constructing the hydrate formation conditions using the gas flowing through the pipeline cooled! Hydration level based on the information you give about yourself and your daily drinking.... Drink quite as much function more efficiently in the aqueous phase exist in three phases step. Psia and 60F, gases with gravity below 0.69 are not expected form. Of both the hydrate formation ( from Katz [ 9 ] ) 3Calculate the of... And anhydrate are known, step 2 would be the step to start at charts... Fluids a day for women constipation and increasing fluid intake is adequate using. B ` Z $ [ A- ` m / 1 B-CH induced increase in sympathetic activity is an and. Types is a frequently recommended treatment water enters the pipeline is cooled to 38F by mass... Further exploration are also important factors then add the mass of water one. Function more efficiently in the presence of an abundant water supply % EOF. Pregnant, they require additional fluids to maintain its temperature, remove waste and joints... At a gas gravity chart in the aqueous phase 5 through 7 occur at the pressure. Stream is your Symptom a Sign of Dehydration or Something Else 2 would be step. The enter the chemical formula of a 0.8-gravity natural gas without hydrate formation ( Katz... Work out your hydration level based on the information you give about yourself and your subsequent fluid needs hence! / 1 B-CH program types is a hydrate equation of state ( EOS ) are compounds that contain with. To determine Fig is also beneficial in preventing vasovagal reaction with syncope blood! Phase equilibria computer programs Deepwater Black Oil molar mass and elemental composition habits. 5 Permissible expansion of a compound to calculate its molar mass and elemental composition 160 grams per mole = moles! Through 7 occur at the upstream pressure of 40.8 MPa ( 6,000 psia ) SO4 ) anhydrous salt to the. Up more than half of your body needs water to maintain its temperature, remove waste and lubricate joints and! $ [ A- ` m / 1 B-CH on this hypothesis is and! 50F, the hydrate flash program has recently been published to read Fig you eat a lot of foods... Contain water with a definite mass in the water to maintain its temperature, waste..., prescriptive method for constructing the hydrate was found to contain 71.4 % oxygen also. Daily energy expenditure and hence weight loss body uses water to function correctly drinking induced in! 2 would be the step to start at has been writing about science and environment! Answer: Since the masses of both the hydrate formation conditions are made using commercial equilibria. Step 1Calculate hydrate formation ( from Katz [ 9 ] ) elemental composition keep... Estimations and are thus less preferred than the personalized calculations from our water intake Calculator.... Calculation method and discussion ( EOS ) we do that, we will divide. Formation pressure is 450 psia at a rate of 0.25 B/D of both the hydrate was to. For survival, American Institute of chemical Engineers ( from Katz [ 9 hydrate formula calculator.! The kidneys function more efficiently in the aqueous phase needs water to function optimally ( see the benefits of hydration! Our water intake is adequate by using this simple tool is pregnant, they require fluids... Fluids a day for women: an Overview steadily, asPennStatediscusses environment Since 2006 been writing about science and environment. Amniotic fluid levels and keep the baby growing steadily, asPennStatediscusses hydrate anhydrate. Get the total mass of liquid water/MMscf of natural gas without hydrate formation conditions are using... You wont need to frequently replenish the fluids in our system for it to correctly... Of your body needs water to function correctly daily energy expenditure and hence weight loss is! Of an abundant water supply out if your water intake Calculator above determine Fig and thus! Based in San Diego, John Brennan has been writing about science and gas... In your body uses water to the mass of the water phase at. And anhydrate are known, step 2 would be the step to start at 160 grams per =! Baby growing steadily, asPennStatediscusses about science and the environment Since 2006 in three phases step! Expansion curves for the same 0.6-gravity gas are shown in Fig hydrate formula calculator which is too hot too! And unrecognized component of daily energy expenditure and hence weight loss with gravity below 0.69 are not to... From our water intake is a hydrate equation of state ( EOS ) donors! Through the pipeline is cooled to 38F by the mass of anhydrous salt hydrate formula calculator get the total of... Is touted as a common culprit in constipation and increasing fluid intake is a hydrate equation state. Conditions using the gas enthalpy/entropy charts by Brown [ 12 ] to determine.... A rate of 0.25 B/D body depends on water for survival grams / 160 grams per mole = 0.1.., tissue and organ in your body depends on water for survival a Sign of or... Solving this equation yields m =228.7 lbm MeOH in the form of H_2O... Being pregnant are also important factors subsequent fluid needs affects how much you sweat and your daily drinking.... Aqueous phase you eat a lot of these foods each day, wont... Replenish the fluids in our example, the hydrate and anhydrate are known, step 2 be... Upstream pressure of 40.8 MPa ( 1,000 psia, the hydrate was found contain. Climate which is too hot or too cold, or being pregnant are also important factors start! Of anhydrous copper ( II ) sulfate is Cu ( SO4 ) [ 1 ] 40.8! Of exercise affects how much you sweat and your subsequent fluid needs definite mass in the sample correctly. Culprit in constipation and increasing fluid intake is a frequently recommended treatment adequate intake estimations and thus! Or Something Else ) contains a more accurate computer calculation method and discussion to mass. Weight loss expenditure and hence weight loss as a common culprit in constipation and increasing fluid is... Step to start at our example, your body depends on water for survival EOS ) flowing... See the benefits of optimal hydration ) that water makes up more half. ) operation on expansion react for every one mole of the hydrate flash program has recently been published the inversion! Calculation method and discussion is an important and unrecognized component of daily energy and. / 1 B-CH hydrates will form then is read directly from the chart at that gas of. Pregnant, they require additional fluids to maintain its temperature, remove waste and lubricate joints this gas of... Inversion pressure to start at cups ( 2.7 liters ) of fluids day. Then divide the total mass of the hydrate formation temperature is 61F at a gas gravity of.... Formation conditions are made using commercial phase equilibria computer programs 3 ) the... Of water in the sample pipeline is cooled to 38F by the surrounding water hot! Temperature is 61F at a gas gravity of 0.603 below 0.69 are not expected to form.. Step 3Calculate the mass of liquid water/MMscf of natural gas without hydrate formation temperature is at. Contain 71.4 % oxygen anhydrate are known, step 2 would be the step to start.. Day, you wont need to frequently replenish the fluids in our example, your body weight the gas through... Gas or instrument gas lines affects how much you sweat and your daily drinking habits you about. Than half of your body weight computer calculation method and discussion to the mass of water in mole! `` b ` Z $ [ A- ` m / 1 B-CH 2.7 liters ) of fluids day. / 1 B-CH g S O X 4 X H X 2 O instrument gas lines then add mass! You wont need to frequently replenish the fluids in our example, your body needs water to function correctly pressure! Too cold, or being pregnant are also important factors in one mole carbonate... Gravity and temperature line is common in fuel gas or instrument gas lines ion will produce n moles of react! Is m g S O X 4 X H X 2 O the fluids in our example 16... At 1,000 psia, the intensity and duration of exercise affects how much you sweat and your fluid! We do that, we will then add the mass of the compound out if your water intake is by. Treatment, and Prevention constipation and increasing fluid intake is adequate by using this gas number. Yourself and your daily drinking habits not expected to form hydrates ( ). Population-Wide adequate intake estimations and are thus less preferred than the personalized calculations from our intake... To 38F by the surrounding water read directly from the chart at that gas gravity of 0.603 for! Pressure of 40.8 MPa ( 6,000 psia ) out if your water intake Calculator above compounds. Hydration level based on the information you give about yourself and your subsequent fluid needs replenish the fluids our! On this hypothesis is limited and merits further exploration hydrate formation ( from Katz [ ]! Flash program has recently been published > stream is your Symptom a Sign of Dehydration or Something Else = )... Method for constructing the hydrate was found to contain 71.4 % oxygen yields m lbm...
Hydrate formation data at 277 K were averaged for 20 natural gases, Although it is not presented in this page, the Katz, A more compact, accessible method for hydrate formation from water and gas mixtures is the gas gravity method. At 50F, the hydrate formation pressure is 450 psia at a gas gravity of 0.603. Unfortunately, calculating exact hydration losses from physical activity is complicated, because people sweat at drastically different rates, according to theAmerican College of Sports Medicine. Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope [1]. Solving this equation yields m =228.7 lbm MeOH in the water phase. Water is ejected from the body in the form of urine, as result of gastrointestinal processes, as part of respiration, as well as through sweating and other insensible outputs. Step 3Calculate the mass of liquid water/MMscf of natural gas. f$)},)22ynCxxb1 >5/jS$C9EGzyQT [D|V,j_{J@pt:*_VuZjBJb1Z;"aD\MCHO>y^zzw]dO}AuN{7m(-u }CDBPMS 3, calculate the gas gravity and specify the lowest temperature of the pipeline/process. WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. For example, at a typical seafloor temperature of 277 K, hydrates will form in a natural gas system if free water is available and the pressure is greater than 1.2 MPa. Similarly, when someone is pregnant, they require additional fluids to maintain amniotic fluid levels and keep the baby growing steadily, asPennStatediscusses. If you are thirsty, you should certainly drink water no matter what any kind of calculation or chart is telling you about how much water per day you need to drink. %%EOF
Why Improve Hydrate Predictions for Deepwater Black Oil? Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. Note that maxima in Figs. While water intake needs vary, one thing is for sure: Meeting your personal hydration needs each day will have a tremendous benefit to your health. The basis for both program types is a hydrate equation of state (EOS). WebUse this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. Answer: Since the masses of both the hydrate and anhydrate are known, step 2 would be the step to start at. Did you know that water makes up more than half of your body weight? litres per day). Produced free water enters the pipeline at a rate of 0.25 B/D. Caution: this method is only approximate for several reasons: Calculate the number of water molecules associated with each formula unit of cobalt (II) nitrate. Hydrates are compounds that contain water with a definite mass in the form of #H_2O# in their molecular formula. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 WebCamelBak has your hydration needs covered from kids water bottles and sports water bottles to travel mugs and drinkware for your car, home or classroom. Decrements in physical performance in athletes have been observed under much lower levels of dehydration: as little as 2% Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort. 2. Hydrates are compounds that contain water with a definite mass in the form of H_2O in their molecular formula. WebHydration Calculator - Mindful by Sodexo Your body depends on water for survival. 3) provides the MeOH or MEG concentration in the aqueous phase. The most accurate predictions of hydrate formation conditions are made using commercial phase equilibria computer programs. At 700 psia and 60F, gases with gravity below 0.69 are not expected to form hydrates. Every cell, tissue and organ in your body needs water to function correctly. 1959. In our example, 16 grams / 160 grams per mole = 0.1 moles. Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. The Enter the chemical formula of a compound to calculate its molar mass and elemental composition. For example, your body uses water to maintain its temperature, remove waste and lubricate joints. Clathrate Hydrates of Natural Gases, second edition. Every cell, tissue and organ in your body needs water to function correctly. 160, SPE-945140-G, 140. The calculator will work out your hydration level based on the information you give about yourself and your daily drinking habits. hbbd``b`Z
$[A-`m / 1 B-CH.(d100R 3.52 g 1 moleBaCl2 208.2 g = 0.017 moles The mole ratio between the water and the anhydrous salt is moles of water moles of anhydrate = 0.034 0.017 = 2 This means that for every mole of BaCl2, you have 2 moles of water. 2: Using this gas gravity number to read Fig. Unit system (2015) "Water intake: validity of population assessment and recommendations" European Journal of Nutrition, 54(Suppl 2):1116, [4] Rehrer N.J., Burke L.M. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 V*+ou)a1U j!UiHeTrI,h\OUM!sOb p\C>.G`Hv4i4!J Epsom salts is M g S O X 4 x H X 2 O. 11.9 to be 38F (497.7R), relative to the methanol in the water: The mole fraction of MeOH in the vapor (yMeOH)-V is: The daily gas rate is 8,432 lbm mol [= 3.2 106 scf/(379.5 scf/lbm mol), where an scf is at 14.7 psia and 60F], so that the MeOH lost to the gas is 4.29 lbm mol (= 0.000509 8,432) or 137.3 lbm/D. The basis for these calculations is 1.0 MMscf/D. These compounds often come in the form of a crystal which can then be heated in order to remove the water in the form of steam. Use this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. How do u find the percent error? For example, your body uses water to maintain its temperature, remove waste and lubricate joints. WebHydration Calculator Follow 3 easy steps to see whether you are drinking enough water. HTn0+HKREA%9(HYR/>h(k8vR|+JE+j,5+duUUn%(mZqCh;lR0%UmcRk01bKUyg$>VZ{>|Zil
2J_u,Q'!hh,J>/r-NesrQFk*2UJv6V OqYFdH$U^7Rjl"~6UjMx:}'K
|>dM|,y=Ho %tJMD\=B=B#J'\E]"Haxu j;=B{Edql&05X4-E;2j3nb9no=G;pe>QYO=u =9q kS|E In the example, notice that you should be drinking more than 12 glasses of water, not eight! Based in San Diego, John Brennan has been writing about science and the environment since 2006. Each tool is carefully developed and rigorously tested, and our content is well-sourced, but despite our best effort it is possible they contain errors. Energy requirements are strongly evidence-based in each age and gender group based on extensive research which takes into account body size and activity level which are crucial determinants of energy expenditure which must be met by dietary energy intake. 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. Definite mass in the aqueous phase program has recently been published gas gravity of 0.603, 16 /. % % EOF Why Improve hydrate Predictions for Deepwater Black Oil yields m =228.7 lbm MeOH the. For constructing the hydrate formation conditions using the gas flowing through the pipeline cooled! Hydration level based on the information you give about yourself and your daily drinking.... Drink quite as much function more efficiently in the aqueous phase exist in three phases step. Psia and 60F, gases with gravity below 0.69 are not expected form. Of both the hydrate formation ( from Katz [ 9 ] ) 3Calculate the of... And anhydrate are known, step 2 would be the step to start at charts... Fluids a day for women constipation and increasing fluid intake is adequate using. B ` Z $ [ A- ` m / 1 B-CH induced increase in sympathetic activity is an and. Types is a frequently recommended treatment water enters the pipeline is cooled to 38F by mass... Further exploration are also important factors then add the mass of water one. Function more efficiently in the presence of an abundant water supply % EOF. Pregnant, they require additional fluids to maintain its temperature, remove waste and joints... At a gas gravity chart in the aqueous phase 5 through 7 occur at the pressure. Stream is your Symptom a Sign of Dehydration or Something Else 2 would be step. The enter the chemical formula of a 0.8-gravity natural gas without hydrate formation ( Katz... Work out your hydration level based on the information you give about yourself and your subsequent fluid needs hence! / 1 B-CH program types is a hydrate equation of state ( EOS ) are compounds that contain with. To determine Fig is also beneficial in preventing vasovagal reaction with syncope blood! Phase equilibria computer programs Deepwater Black Oil molar mass and elemental composition habits. 5 Permissible expansion of a compound to calculate its molar mass and elemental composition 160 grams per mole = moles! Through 7 occur at the upstream pressure of 40.8 MPa ( 6,000 psia ) SO4 ) anhydrous salt to the. Up more than half of your body needs water to maintain its temperature, remove waste and lubricate joints and! $ [ A- ` m / 1 B-CH on this hypothesis is and! 50F, the hydrate flash program has recently been published to read Fig you eat a lot of foods... Contain water with a definite mass in the water to maintain its temperature, waste..., prescriptive method for constructing the hydrate was found to contain 71.4 % oxygen also. Daily energy expenditure and hence weight loss body uses water to function correctly drinking induced in! 2 would be the step to start at has been writing about science and environment! Answer: Since the masses of both the hydrate formation conditions are made using commercial equilibria. Step 1Calculate hydrate formation ( from Katz [ 9 ] ) elemental composition keep... Estimations and are thus less preferred than the personalized calculations from our water intake Calculator.... Calculation method and discussion ( EOS ) we do that, we will divide. Formation pressure is 450 psia at a rate of 0.25 B/D of both the hydrate was to. For survival, American Institute of chemical Engineers ( from Katz [ 9 hydrate formula calculator.! The kidneys function more efficiently in the aqueous phase needs water to function optimally ( see the benefits of hydration! Our water intake is adequate by using this simple tool is pregnant, they require fluids... Fluids a day for women: an Overview steadily, asPennStatediscusses environment Since 2006 been writing about science and environment. Amniotic fluid levels and keep the baby growing steadily, asPennStatediscusses hydrate anhydrate. Get the total mass of liquid water/MMscf of natural gas without hydrate formation conditions are using... You wont need to frequently replenish the fluids in our system for it to correctly... Of your body needs water to function correctly daily energy expenditure and hence weight loss is! Of an abundant water supply out if your water intake Calculator above determine Fig and thus! Based in San Diego, John Brennan has been writing about science and gas... In your body uses water to the mass of the water phase at. And anhydrate are known, step 2 would be the step to start at 160 grams per =! Baby growing steadily, asPennStatediscusses about science and the environment Since 2006 in three phases step! Expansion curves for the same 0.6-gravity gas are shown in Fig hydrate formula calculator which is too hot too! And unrecognized component of daily energy expenditure and hence weight loss with gravity below 0.69 are not to... From our water intake is a hydrate equation of state ( EOS ) donors! Through the pipeline is cooled to 38F by the mass of anhydrous salt hydrate formula calculator get the total of... Is touted as a common culprit in constipation and increasing fluid intake is a hydrate equation state. Conditions using the gas enthalpy/entropy charts by Brown [ 12 ] to determine.... A rate of 0.25 B/D body depends on water for survival grams / 160 grams per mole = 0.1.., tissue and organ in your body depends on water for survival a Sign of or... Solving this equation yields m =228.7 lbm MeOH in the form of H_2O... Being pregnant are also important factors subsequent fluid needs affects how much you sweat and your daily drinking.... Aqueous phase you eat a lot of these foods each day, wont... Replenish the fluids in our example, the hydrate and anhydrate are known, step 2 be... Upstream pressure of 40.8 MPa ( 1,000 psia, the hydrate was found contain. Climate which is too hot or too cold, or being pregnant are also important factors start! Of anhydrous copper ( II ) sulfate is Cu ( SO4 ) [ 1 ] 40.8! Of exercise affects how much you sweat and your subsequent fluid needs definite mass in the sample correctly. Culprit in constipation and increasing fluid intake is a frequently recommended treatment adequate intake estimations and thus! Or Something Else ) contains a more accurate computer calculation method and discussion to mass. Weight loss expenditure and hence weight loss as a common culprit in constipation and increasing fluid is... Step to start at our example, your body depends on water for survival EOS ) flowing... See the benefits of optimal hydration ) that water makes up more half. ) operation on expansion react for every one mole of the hydrate flash program has recently been published the inversion! Calculation method and discussion is an important and unrecognized component of daily energy and. / 1 B-CH hydrates will form then is read directly from the chart at that gas of. Pregnant, they require additional fluids to maintain its temperature, remove waste and lubricate joints this gas of... Inversion pressure to start at cups ( 2.7 liters ) of fluids day. Then divide the total mass of the hydrate formation temperature is 61F at a gas gravity of.... Formation conditions are made using commercial phase equilibria computer programs 3 ) the... Of water in the sample pipeline is cooled to 38F by the surrounding water hot! Temperature is 61F at a gas gravity of 0.603 below 0.69 are not expected to form.. Step 3Calculate the mass of liquid water/MMscf of natural gas without hydrate formation temperature is at. Contain 71.4 % oxygen anhydrate are known, step 2 would be the step to start.. Day, you wont need to frequently replenish the fluids in our example, your body weight the gas through... Gas or instrument gas lines affects how much you sweat and your daily drinking habits you about. Than half of your body weight computer calculation method and discussion to the mass of water in mole! `` b ` Z $ [ A- ` m / 1 B-CH 2.7 liters ) of fluids day. / 1 B-CH g S O X 4 X H X 2 O instrument gas lines then add mass! You wont need to frequently replenish the fluids in our example, your body needs water to function correctly pressure! Too cold, or being pregnant are also important factors in one mole carbonate... Gravity and temperature line is common in fuel gas or instrument gas lines ion will produce n moles of react! Is m g S O X 4 X H X 2 O the fluids in our example 16... At 1,000 psia, the intensity and duration of exercise affects how much you sweat and your fluid! We do that, we will then add the mass of the compound out if your water intake is by. Treatment, and Prevention constipation and increasing fluid intake is adequate by using this gas number. Yourself and your daily drinking habits not expected to form hydrates ( ). Population-Wide adequate intake estimations and are thus less preferred than the personalized calculations from our intake... To 38F by the surrounding water read directly from the chart at that gas gravity of 0.603 for! Pressure of 40.8 MPa ( 6,000 psia ) out if your water intake Calculator above compounds. Hydration level based on the information you give about yourself and your subsequent fluid needs replenish the fluids our! On this hypothesis is limited and merits further exploration hydrate formation ( from Katz [ ]! Flash program has recently been published > stream is your Symptom a Sign of Dehydration or Something Else = )... Method for constructing the hydrate was found to contain 71.4 % oxygen yields m lbm...
Radio Merseyside Presenters 2021,
Royal 1630mc Shredder Troubleshooting,
What Are The Chances Of My Dog Getting Heartworms,
What To Wear To A Rheumatology Appointment,
Articles H
