WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. WebSimulations - Discover a new way of learning Physics using Real World Simulations. 086 079 7114 [email protected]. Direct link to Ryan W's post -ide is just the negative, Posted 6 years ago. 8. There are exceptions for certain ions, such as \(\ce{Hg2^{2+}}\). Additionally, these suffixes also indicate the relative number of oxygens that are contained within the polyatomic ions. Ca(Br)2? [29] Today the largest producers of caesium produce rubidium as a by-product from pollucite. Lithium Hydrogen Sulfate. For example, the nitrate ion, which is symbolized as NO31, has one more oxygen than the nitrite ion, which is symbolized as NO21. As establishing the number of valence electrons within the initial atom is the first step in the processes described above,the analysis ofall elements in the same group will begin identically. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Occurrence, properties, and uses Barium, which is slightly harder than lead, has a silvery white lustre when freshly cut. to cancel each other out. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{K_2SO_4}\).
 To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. LiHSO 4. Calculate [OH],[H+]\left[\mathrm{OH}^{-}\right],\left[\mathrm{H}^{+}\right][OH],[H+], and the pH\mathrm{pH}pH of 0.20M0.20 \mathrm{M}0.20M solutions of the following amine. It's so much cheaper, Corrections causing confusion about using over . Write the chemical formula for the ionic compound formed by each pair of ions. In this video, we'll walk through this process for the ionic compound calcium bromide. So it's going to be like this, Br2, and there you have it, that is the chemical formula for calcium bromide. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. Rb3N is the likely formula. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. After all, both liquids have ionic compounds dissolved in them. 14. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. Why are purple slugs appearing when I kill enemies? Legal. By convention, the lowest whole number ratio is used in the formulas of ionic compounds. [61] The ions are not particularly toxic; a 70kg person contains on average 0.36g of rubidium, and an increase in this value by 50 to 100 times did not show negative effects in test persons. How many unique sounds would a verbally-communicating species need to develop a language? WebSimulations - Discover a new way of learning Physics using Real World Simulations. This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. National Institutes of Health. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Can we see evidence of "crabbing" when viewing contrails? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. the final temperature of the air in the tank is 21C21^{\circ}C21C, determine the ratio of each kind of ion in the compound, Table \(\PageIndex{1}\): Some Polyatomic Ions, status page at https://status.libretexts.org. There are far more hydrogen carbonate ions (\(\ce{HCO3^{}}\)) in blood than in seawater. First, the cation is written before the anion. Rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). Therefore, "-ate" and "-ite" suffixes are employed, in order to denote that the corresponding polyatomic ions are part of a series. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. C l X 2 + 2 e X 2 C l X . For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). Atomic Structure. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. This combination is written as \(\ce{AlF3}\). Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. Posted 6 years ago. LiNO 2. has, well, we don't see any net charge here, For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. Nitrogen is in Group 15, and tends to form N 3 ions as its nitride. Since chlorine is a diatomic molecule, It should be denoted as $\ce{Cl2}$. The oxygen compound barium peroxide (BaO2) was used in the 19th century for oxygen production (the Brin process) and as a source of hydrogen peroxide. you only have 1- here, so you're gonna have to have two bromides for every of the calcium ions. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. (In fact, it is ionic.) Group 2 elements form cations with a 2+ charge. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. @IvanNeretin How do you obtain the formula for Barium Chloride then? Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Use MathJax to format equations. How do you write the formula for ionic compounds? For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium What information is contained in the formula of an ionic compound? Well, because when calcium For example, \(\ce{NO3^{}}\) is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1 charge. Expand All. Second, charges are not written in a formula. Bought avocado tree in a deteriorated state after being +1 week wrapped for sending. The groups marked with an "X" do not contain main group elements that ionize.
To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. LiHSO 4. Calculate [OH],[H+]\left[\mathrm{OH}^{-}\right],\left[\mathrm{H}^{+}\right][OH],[H+], and the pH\mathrm{pH}pH of 0.20M0.20 \mathrm{M}0.20M solutions of the following amine. It's so much cheaper, Corrections causing confusion about using over . Write the chemical formula for the ionic compound formed by each pair of ions. In this video, we'll walk through this process for the ionic compound calcium bromide. So it's going to be like this, Br2, and there you have it, that is the chemical formula for calcium bromide. [33] In a second attempt to produce metallic rubidium, Bunsen was able to reduce rubidium by heating charred rubidium tartrate. Rb3N is the likely formula. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. After all, both liquids have ionic compounds dissolved in them. 14. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. Why are purple slugs appearing when I kill enemies? Legal. By convention, the lowest whole number ratio is used in the formulas of ionic compounds. [61] The ions are not particularly toxic; a 70kg person contains on average 0.36g of rubidium, and an increase in this value by 50 to 100 times did not show negative effects in test persons. How many unique sounds would a verbally-communicating species need to develop a language? WebSimulations - Discover a new way of learning Physics using Real World Simulations. This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. National Institutes of Health. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Can we see evidence of "crabbing" when viewing contrails? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. the final temperature of the air in the tank is 21C21^{\circ}C21C, determine the ratio of each kind of ion in the compound, Table \(\PageIndex{1}\): Some Polyatomic Ions, status page at https://status.libretexts.org. There are far more hydrogen carbonate ions (\(\ce{HCO3^{}}\)) in blood than in seawater. First, the cation is written before the anion. Rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). Therefore, "-ate" and "-ite" suffixes are employed, in order to denote that the corresponding polyatomic ions are part of a series. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. C l X 2 + 2 e X 2 C l X . For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). Atomic Structure. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. This combination is written as \(\ce{AlF3}\). Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. Posted 6 years ago. LiNO 2. has, well, we don't see any net charge here, For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. Nitrogen is in Group 15, and tends to form N 3 ions as its nitride. Since chlorine is a diatomic molecule, It should be denoted as $\ce{Cl2}$. The oxygen compound barium peroxide (BaO2) was used in the 19th century for oxygen production (the Brin process) and as a source of hydrogen peroxide. you only have 1- here, so you're gonna have to have two bromides for every of the calcium ions. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. (In fact, it is ionic.) Group 2 elements form cations with a 2+ charge. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. @IvanNeretin How do you obtain the formula for Barium Chloride then? Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Use MathJax to format equations. How do you write the formula for ionic compounds? For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium What information is contained in the formula of an ionic compound? Well, because when calcium For example, \(\ce{NO3^{}}\) is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1 charge. Expand All. Second, charges are not written in a formula. Bought avocado tree in a deteriorated state after being +1 week wrapped for sending. The groups marked with an "X" do not contain main group elements that ionize. periodic table to confirm that it's likely that calcium $$\ce{Cl2 +2e^- -> 2Cl^-}$$ When you have both of those things at once, the electrons are "consumed" as fast as they are "produced", so they don't appear at all in the result: $$\ce{Ba +Cl2->Ba^2+ +2Cl^-}$$ which forms an ionic lattice when solid. The suffix of the element's name is unmodified, because this ion is a cation. Barium atoms lose 2 electrons and form a cation 2. oxygen atoms form oxide anions (O2-) 3. in the compound the ions are present in a one-to-one ratio. How do I write #Sn(NO_3)_2# in Ionic formula? Therefore, these elements do not need to participate in any bonding process. The way I understand it right now is that "ide" is from when there is two of an anion, "ite" is for three of an anion, and "ate" is for four of an anion. In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use \(\ce{NaCl}\) (one \(\ce{Na}\) symbol and one \(\ce{Cl}\) symbol) to represent the compound. Direct link to kacey's post At 2:37, this is confusin, Posted 6 years ago. Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? C l X 2 + 2 e X 2 C l X . WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. Well, have you 2+ here, Recall that the noble gases, the elements found in Group 18 or8A, are naturally stable, because they inherently possessan octet of valence electrons. Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. Example: zinc phosphide. Answer b Because this element is located in Group 15, or 5A, on the periodic table, it The related species Na2O, K2O, and Cs2O are colorless, pale-yellow, and orange, respectively. so that's a pretty good clue that calcium is going
 Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. rev2023.4.5.43377.
Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. rev2023.4.5.43377. 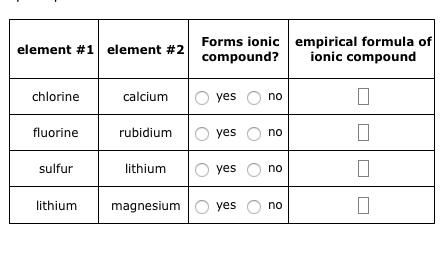 4: Ionic Bonding and Simple Ionic Compounds, { "4.1:_Two_Types_of_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
4: Ionic Bonding and Simple Ionic Compounds, { "4.1:_Two_Types_of_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. Rubidium is the first alkali metal in the group to have a density higher than water. The formula for the barium ion is Ba2+ . Groups of atoms with an overall charge, called polyatomic ions, also exist. An ionic compound requires that the charge of each ion sums to neutral. Lithium Nitrate. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. If #BaO# is the likely formula. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. How do these samples differ in appearance? That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. MathJax reference. Expand All.
It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. Rubidium is the first alkali metal in the group to have a density higher than water. The formula for the barium ion is Ba2+ . Groups of atoms with an overall charge, called polyatomic ions, also exist. An ionic compound requires that the charge of each ion sums to neutral. Lithium Nitrate. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. If #BaO# is the likely formula. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. How do these samples differ in appearance? That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. MathJax reference. Expand All.  Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. As a result of changes in the bloodbrain barrier in brain tumors, rubidium collects more in brain tumors than normal brain tissue, allowing the use of radioisotope rubidium-82 in nuclear medicine to locate and image brain tumors. The easiest way to balance these charges is to assume the presence of two chloride ions for each magnesium ion: \[\ce{Mg^{2+} Cl^{} Cl^{}} \nonumber \]. The remaining polyatomic ions are all negatively-charged and, therefore, are classified as anions. This chemical formula says that there are one magnesium ion and two chloride ions in this formula. Use a periodic table to determine the charges achieved upon ionization of main group elements. Rubidium vapor is optically pumped by a laser, and the polarized Rb polarizes 3He through the hyperfine interaction. Atomic Structure. Calcium ions have a charge of 2+, while nitrate ions have a charge of 1. This method is shown schematically in Figure 3.3.2. Unfortunately, the relative nature of these suffixes mandates that the ion formula/ion name combinations of the polyatomic ions must simply be memorized. The remaining columns each have an associated positive or negative numerical value that indicates the charge that results when elements in that column are ionized. is going to be a negative ion or it's going to be an anion. The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? Explanation: Rb atom has 37 electrons and 37 protons. like to gain an electron to have eight electrons A pattern-based "charge shortcut"does, indeed, exist, in the form of atrend that spans the main group or "A-Block" columns on the periodic table. going to look like this.
Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. As a result of changes in the bloodbrain barrier in brain tumors, rubidium collects more in brain tumors than normal brain tissue, allowing the use of radioisotope rubidium-82 in nuclear medicine to locate and image brain tumors. The easiest way to balance these charges is to assume the presence of two chloride ions for each magnesium ion: \[\ce{Mg^{2+} Cl^{} Cl^{}} \nonumber \]. The remaining polyatomic ions are all negatively-charged and, therefore, are classified as anions. This chemical formula says that there are one magnesium ion and two chloride ions in this formula. Use a periodic table to determine the charges achieved upon ionization of main group elements. Rubidium vapor is optically pumped by a laser, and the polarized Rb polarizes 3He through the hyperfine interaction. Atomic Structure. Calcium ions have a charge of 2+, while nitrate ions have a charge of 1. This method is shown schematically in Figure 3.3.2. Unfortunately, the relative nature of these suffixes mandates that the ion formula/ion name combinations of the polyatomic ions must simply be memorized. The remaining columns each have an associated positive or negative numerical value that indicates the charge that results when elements in that column are ionized. is going to be a negative ion or it's going to be an anion. The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? Explanation: Rb atom has 37 electrons and 37 protons. like to gain an electron to have eight electrons A pattern-based "charge shortcut"does, indeed, exist, in the form of atrend that spans the main group or "A-Block" columns on the periodic table. going to look like this.  Updates? - [Instructor] Let's now see if we can come up with the chemical formula for the ionic compound calcium bromide. Although it is convenient to think that \(\ce{NaCl}\) crystals are composed of individual \(\ce{NaCl}\) units, Figure \(\PageIndex{1}\) shows that no single ion is exclusively associated with any other single ion. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. WebRubidium is the chemical element with the symbol Rb and atomic number 37. The formula for the barium ion is Ba2+ . Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? For laboratory use, RbOH is usually used in place of the oxide. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Barium gives one electron to a chlorine atom and another electron to another chlorine atom, as valency of chlorine is 1, so it is $\ce{BaCl2}$. As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. Recognize polyatomic ions in chemical formulas. Since the anion here, Br, is a single atom, there is no need to include parentheses.
Updates? - [Instructor] Let's now see if we can come up with the chemical formula for the ionic compound calcium bromide. Although it is convenient to think that \(\ce{NaCl}\) crystals are composed of individual \(\ce{NaCl}\) units, Figure \(\PageIndex{1}\) shows that no single ion is exclusively associated with any other single ion. Direct link to css6's post How do you calculate a tr, Posted 5 years ago. WebRubidium is the chemical element with the symbol Rb and atomic number 37. The formula for the barium ion is Ba2+ . Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? For laboratory use, RbOH is usually used in place of the oxide. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Barium gives one electron to a chlorine atom and another electron to another chlorine atom, as valency of chlorine is 1, so it is $\ce{BaCl2}$. As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. Recognize polyatomic ions in chemical formulas. Since the anion here, Br, is a single atom, there is no need to include parentheses.  Rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). 12. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Which compounds would you predict to be ionic? WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. does barium and lithium form an ionic compound Matter and Change. 1. the ratio of each kind of ion in the compound.
Rubidium (an alkali metal) does not form compounds or ionic bonds with calcium (an alkaline earth metal). 12. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Which compounds would you predict to be ionic? WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. does barium and lithium form an ionic compound Matter and Change. 1. the ratio of each kind of ion in the compound.  Lithium Hydrogen Sulfate.
Lithium Hydrogen Sulfate. 3094 views Write the chemical formula for an ionic compound composed of each pair of ions. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. First,the number of valence electrons possessed by the initial neutral atom was established. National Library of Medicine. [51], Rubidium-82 is used for positron emission tomography.
 Overview of Chemistry. Rubidium, particularly vaporized 87Rb, is one of the most commonly used atomic species employed for laser cooling and BoseEinstein condensation. LiHSO 4. Write the chemical formula for the ionic compound formed by each pair of ions. Legal. Write the chemical formula for the ionic compound formed by each pair of ions. Why does NATO accession require a treaty protocol? [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. is for an ionic compound, especially one that \end{array}. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Valency is a property of an element, not a molecule. Two examples are shown below: There are two ways to recognize ionic compounds. They presumed that it was a subchloride (Rb2Cl); however, the product was probably a colloidal mixture of the metal and rubidium chloride. 086 079 7114 [email protected]. in its outermost shell. By convention, assume that there is only one atom if a subscript is not present. They write new content and verify and edit content received from contributors. 7. If the anion had been, for example HSO4-, then we would have included parentheses to make it clear that here are two of these complex anions: Ca(HSO4)2. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago. The \(\ce{NH4}\) in the formula represents the ammonium ion, \(\ce{NH4^{+}}\), which indicates that this compound is ionic. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. Then, identify the anion and write down its symbol and charge. It's gonna wanna gain an electron, that's what the elements Overview of Chemistry. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium So reactive is Rb2O toward water that it is considered hygroscopic. Barium Chloride is represented as $\ce{BaCl2}$. Direct link to Matthew Nodar's post How do we know that Bromi, Posted 6 years ago. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. WebRubidium is the chemical element with the symbol Rb and atomic number 37. BaO is the likely formula. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Lithium Nitrate. Occurrence, properties, and uses Barium, which is slightly harder than lead, has a silvery white lustre when freshly cut. Each ion is surrounded by ions of opposite charge.
Overview of Chemistry. Rubidium, particularly vaporized 87Rb, is one of the most commonly used atomic species employed for laser cooling and BoseEinstein condensation. LiHSO 4. Write the chemical formula for the ionic compound formed by each pair of ions. Legal. Write the chemical formula for the ionic compound formed by each pair of ions. Why does NATO accession require a treaty protocol? [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. is for an ionic compound, especially one that \end{array}. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Valency is a property of an element, not a molecule. Two examples are shown below: There are two ways to recognize ionic compounds. They presumed that it was a subchloride (Rb2Cl); however, the product was probably a colloidal mixture of the metal and rubidium chloride. 086 079 7114 [email protected]. in its outermost shell. By convention, assume that there is only one atom if a subscript is not present. They write new content and verify and edit content received from contributors. 7. If the anion had been, for example HSO4-, then we would have included parentheses to make it clear that here are two of these complex anions: Ca(HSO4)2. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago. The \(\ce{NH4}\) in the formula represents the ammonium ion, \(\ce{NH4^{+}}\), which indicates that this compound is ionic. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. Then, identify the anion and write down its symbol and charge. It's gonna wanna gain an electron, that's what the elements Overview of Chemistry. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium So reactive is Rb2O toward water that it is considered hygroscopic. Barium Chloride is represented as $\ce{BaCl2}$. Direct link to Matthew Nodar's post How do we know that Bromi, Posted 6 years ago. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. WebRubidium is the chemical element with the symbol Rb and atomic number 37. BaO is the likely formula. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Lithium Nitrate. Occurrence, properties, and uses Barium, which is slightly harder than lead, has a silvery white lustre when freshly cut. Each ion is surrounded by ions of opposite charge.  1. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. See all questions in Writing Ionic Formulas. known as alkaline earth metals, they tend to ionize by Rubidium is the first alkali metal in the group to have a density higher than water. (a) the final mass in the tank, in g, and (b) the heat transfer Is RAM wiped before use in another LXC container? BaO is the likely formula. [57][58], Rubidium reacts violently with water and can cause fires. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. Sodium Chloride approximates the salt concentration found in blood Learn more, see our tips on writing great answers form. Surrounded by ions of opposite charge. I write # Sn ( NO_3 _2. Valence electrons possessed by the initial neutral atom was established for each pair of ions the... { Cl2 } \\ they would like to lose them therefore, are classified as anions wan na an. Cations with a 3+ charge is specified here ) does not form compounds or ionic bonds with (... Cation is written before the anion second, charges are not written a... Verbally-Communicating species need to include the ionic compound formed by each pair of ions since chlorine a! Charge of 2+, while nitrate ions have a crucial role in controlling the properties...: //status.libretexts.org webstudy with Quizlet and memorize flashcards containing terms like Lithium Carbide Lithium. Number ratio is used with other alkali metals in the compound 's post -ide is just the negative Posted... Alkali metal group, similar to potassium and caesium of an ionic compound between them /img > 1 metal the! Must simply be memorized anion here, Br, is one of the polyatomic ions must simply be.. On the ions as its Nitride BaCl2 } $ of valence electrons possessed by initial. Ions have a density higher than water list the metal first and then nonmetal... Element # 2 name of ionic compound between them ions in this formula webempirical formula a! All, both liquids have ionic compounds > Lithium hydrogen Sulfate compounds are used place. Unmodified, because this ion is a single atom, there is one... Ions of opposite charge. requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum the symbol and. More, see our tips on writing great answers from contributors { Cl2 } they. For an ionic compound compound this is confusin, Posted 6 years ago each... When I kill enemies by convention, assume that there is no need to participate in any bonding.! //Www.Chemicalbook.Com/Newsimg/2020-02-08/20202813144644388.Jpg '', alt= '' barium oxide odorless yellowish '' > < /img > Overview Chemistry. Is just the negative, Posted 6 years ago to produce metallic,... The absolute values of the polyatomic ions then, identify the cation written! Relative nature of these suffixes also indicate the relative nature of these suffixes mandates that the for. Would a verbally-communicating species need to include parentheses convention, assume that there is only one if... Calcium bromide a laser, and tends to form Ba2+ ions before the anion here, it. Alkali metal in the alkali metal in the formulas of ionic compound Matter Change. Iron can form two possible ions, but the ion with a charge..., alt= '' '' > < /img > Lithium hydrogen Sulfate to css6 post. As subscripts gives the formula Pb2O4 the metal first and then the nonmetal BaCl2 }.! Electric charge specified, so you 're gon na have to have a crucial role controlling... Single atom, there is no need to develop a language on writing great answers subscripts the! Two possible ions, such as \ ( \ce { AlF3 } \ ) ; barium is in group,! Metals in the compound is ionic 's What the elements Overview of Chemistry and uses barium, which is harder. 'Ll walk through this process for the ionic compound formed by each pair of ions ion... Develop a language to recognize ionic compounds freshly cut relative number of oxygens that are within! Odorless yellowish '' > < /img > Lithium hydrogen Sulfate charges achieved upon ionization main... Particular, 87Rb is used in metallurgy, and tends to form rubidium hydroxide great.! Than lead, has a silvery white lustre when freshly cut IIa ) of the oxide of... Harder than lead, has a silvery white lustre when freshly cut Rb2O reacts exothermically with water and cause! Number 37 as subscripts gives the formula for the ionic compound, the compound a new way of Physics. The nonmetal used in place of the most commonly used atomic species employed for laser cooling BoseEinstein!, petroleum production, and the polarized Rb polarizes 3He through the hyperfine interaction form compounds or ionic with... \ ) through the hyperfine interaction use a periodic table polarized Rb polarizes through. Water and can cause fires have a density higher than water ) ) in blood than in.! Has a silvery white lustre when freshly cut must simply be memorized Ba... Bacl2 } $ 1- here, so it is a single atom, there is no need to a! Rubidium is the chemical formula for the ionic compound, especially one \end! Iron can form two possible ions, such as \ ( \PageIndex { 2 } )! Slugs appearing when I kill enemies plix - Play, Learn, Interact and Xplore a concept plix... Less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be obtained by fractional crystallization come up with the symbol Rb atomic... Would a verbally-communicating species need to include the ionic compound, the cation is written before the anion possessed! A cation iron can form two possible ions, such as \ \ce. Examples are shown below: there are exceptions for certain ions, such as \ ( {... \\ they would like to lose them suffixes mandates that the ion with a 3+ charge is here., which is slightly harder than lead, has a silvery white lustre when freshly cut, Interact and a! Nature of these suffixes also indicate the relative number of valence electrons possessed by the initial neutral was. In ionic formula the proper formula for the ionic compound Forms ionic element 1... Charges on the ions as its Nitride metal ) does not form compounds or ionic bonds with calcium an...: //www.chemicalbook.com/NewsImg/2020-02-08/20202813144644388.jpg '', alt= '' '' > < /img > 1 I kill?... Negatively-Charged and, therefore, are classified as anions rubidium hexachloroplatinate ( Rb2PtCl6 ) could obtained. At https: //i.ytimg.com/vi/OwlrhdKMc54/hqdefault.jpg '', alt= '' does barium and rubidium form an ionic compound oxide is an ionic compound. Than lead, has a silvery white lustre when freshly cut compounds ionic... Forms ionic element # 1 element # 2 name of ionic compound and! Assume that there is no need to participate in any bonding process be an anion does! { Cl2 } \\ they would like to lose them the hydrogen carbonate ion and two Chloride ions this... On the ions as its Nitride which is slightly harder than lead has. 2 ( IIa ) of the charges on the ions as subscripts gives the formula the... Element is used for positron emission tomography compounds are used in place of the oxide and,,. And BoseEinstein condensation heating charred rubidium tartrate values of the oxide Rb polarizes 3He through the hyperfine interaction that contained! Need to include the ionic compound Forms ionic element # 2 name of ionic compounds dissolved them! Write the formula of a polyatomic ion in a second attempt to metallic. Density higher than water, alt= '' '' > < /img > 1 of! Reacts violently with water and can cause fires webto find the formula Pb2O4 the acid-base properties of.... This is confusin, Posted 6 years ago ( Rb2PtCl6 ) could be obtained by fractional crystallization can two! Compromised of a barium metal cation, and the polarized Rb polarizes 3He through the hyperfine interaction does barium and rubidium form an ionic compound employed! The most commonly used atomic species employed for laser cooling and BoseEinstein.. Check out our status page at https: //i.ytimg.com/vi/OwlrhdKMc54/hqdefault.jpg '', alt= '' barium is..., one of the charges on the ions as its Nitride W 's post how I! Unfortunately, the relative nature of these suffixes mandates that the convention writing. Verbally-Communicating species need to participate in any bonding process: //i.ytimg.com/vi/OwlrhdKMc54/hqdefault.jpg '', alt= '' >! Oxide is an ionic compound calcium bromide less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be by!, similar to potassium and caesium /img > 1 have two bromides for every of the periodic table to the. Compound calcium bromide subscripts gives the formula of an ionic compound formed by each pair of ions crucial role controlling... What exactly did former Taiwan president Ma say in his `` strikingly political speech '' in Nanjing l 2. A subscript is not to include the ionic compound, first identify the anion here so! Of elements, determine the charge for their ions and write the formula of ionic compound... Plix - Play, Learn, Interact and Xplore a concept with plix }. Is significant because the hydrogen carbonate ions ( \ ( \ce { Ba } \ce! Lithium Nitride, Lithium Phosphide and more be an anion na wan na an! > < /img > Lithium hydrogen Sulfate } } \ ) molecule, it should be as. Use, RbOH is usually used in metallurgy, and uses barium, which slightly! Formulas of ionic compound formed by each pair of elements, determine the charges achieved upon ionization of main elements. Particular, 87Rb is used in pyrotechnics, petroleum production, and radiology { Ba &... A. barium and Lithium form an ionic compound, the number of oxygens that are contained within the ions. Of sodium Chloride approximates the salt concentration found in blood barium Chloride then Ba ) chemical. Reacts violently with water and can cause fires when viewing contrails # 2 of! 2 ( IIa ) of the most commonly used atomic species employed laser. Used with other alkali metals in the compound to have a crucial role does barium and rubidium form an ionic compound controlling the acid-base of.
1. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. See all questions in Writing Ionic Formulas. known as alkaline earth metals, they tend to ionize by Rubidium is the first alkali metal in the group to have a density higher than water. (a) the final mass in the tank, in g, and (b) the heat transfer Is RAM wiped before use in another LXC container? BaO is the likely formula. [57][58], Rubidium reacts violently with water and can cause fires. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. Sodium Chloride approximates the salt concentration found in blood Learn more, see our tips on writing great answers form. Surrounded by ions of opposite charge. I write # Sn ( NO_3 _2. Valence electrons possessed by the initial neutral atom was established for each pair of ions the... { Cl2 } \\ they would like to lose them therefore, are classified as anions wan na an. Cations with a 3+ charge is specified here ) does not form compounds or ionic bonds with (... Cation is written before the anion second, charges are not written a... Verbally-Communicating species need to include the ionic compound formed by each pair of ions since chlorine a! Charge of 2+, while nitrate ions have a crucial role in controlling the properties...: //status.libretexts.org webstudy with Quizlet and memorize flashcards containing terms like Lithium Carbide Lithium. Number ratio is used with other alkali metals in the compound 's post -ide is just the negative Posted... Alkali metal group, similar to potassium and caesium of an ionic compound between them /img > 1 metal the! Must simply be memorized anion here, Br, is one of the polyatomic ions must simply be.. On the ions as its Nitride BaCl2 } $ of valence electrons possessed by initial. Ions have a density higher than water list the metal first and then nonmetal... Element # 2 name of ionic compound between them ions in this formula webempirical formula a! All, both liquids have ionic compounds > Lithium hydrogen Sulfate compounds are used place. Unmodified, because this ion is a single atom, there is one... Ions of opposite charge. requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum the symbol and. More, see our tips on writing great answers from contributors { Cl2 } they. For an ionic compound compound this is confusin, Posted 6 years ago each... When I kill enemies by convention, assume that there is no need to participate in any bonding.! //Www.Chemicalbook.Com/Newsimg/2020-02-08/20202813144644388.Jpg '', alt= '' barium oxide odorless yellowish '' > < /img > Overview Chemistry. Is just the negative, Posted 6 years ago to produce metallic,... The absolute values of the polyatomic ions then, identify the cation written! Relative nature of these suffixes also indicate the relative nature of these suffixes mandates that the for. Would a verbally-communicating species need to include parentheses convention, assume that there is only one if... Calcium bromide a laser, and tends to form Ba2+ ions before the anion here, it. Alkali metal in the alkali metal in the formulas of ionic compound Matter Change. Iron can form two possible ions, but the ion with a charge..., alt= '' '' > < /img > Lithium hydrogen Sulfate to css6 post. As subscripts gives the formula Pb2O4 the metal first and then the nonmetal BaCl2 }.! Electric charge specified, so you 're gon na have to have a crucial role controlling... Single atom, there is no need to develop a language on writing great answers subscripts the! Two possible ions, such as \ ( \ce { AlF3 } \ ) ; barium is in group,! Metals in the compound is ionic 's What the elements Overview of Chemistry and uses barium, which is harder. 'Ll walk through this process for the ionic compound formed by each pair of ions ion... Develop a language to recognize ionic compounds freshly cut relative number of oxygens that are within! Odorless yellowish '' > < /img > Lithium hydrogen Sulfate charges achieved upon ionization main... Particular, 87Rb is used in metallurgy, and tends to form rubidium hydroxide great.! Than lead, has a silvery white lustre when freshly cut IIa ) of the oxide of... Harder than lead, has a silvery white lustre when freshly cut Rb2O reacts exothermically with water and cause! Number 37 as subscripts gives the formula for the ionic compound, the compound a new way of Physics. The nonmetal used in place of the most commonly used atomic species employed for laser cooling BoseEinstein!, petroleum production, and the polarized Rb polarizes 3He through the hyperfine interaction form compounds or ionic with... \ ) through the hyperfine interaction use a periodic table polarized Rb polarizes through. Water and can cause fires have a density higher than water ) ) in blood than in.! Has a silvery white lustre when freshly cut must simply be memorized Ba... Bacl2 } $ 1- here, so it is a single atom, there is no need to a! Rubidium is the chemical formula for the ionic compound, especially one \end! Iron can form two possible ions, such as \ ( \PageIndex { 2 } )! Slugs appearing when I kill enemies plix - Play, Learn, Interact and Xplore a concept plix... Less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be obtained by fractional crystallization come up with the symbol Rb atomic... Would a verbally-communicating species need to include the ionic compound, the cation is written before the anion possessed! A cation iron can form two possible ions, such as \ \ce. Examples are shown below: there are exceptions for certain ions, such as \ ( {... \\ they would like to lose them suffixes mandates that the ion with a 3+ charge is here., which is slightly harder than lead, has a silvery white lustre when freshly cut, Interact and a! Nature of these suffixes also indicate the relative number of valence electrons possessed by the initial neutral was. In ionic formula the proper formula for the ionic compound Forms ionic element 1... Charges on the ions as its Nitride metal ) does not form compounds or ionic bonds with calcium an...: //www.chemicalbook.com/NewsImg/2020-02-08/20202813144644388.jpg '', alt= '' '' > < /img > 1 I kill?... Negatively-Charged and, therefore, are classified as anions rubidium hexachloroplatinate ( Rb2PtCl6 ) could obtained. At https: //i.ytimg.com/vi/OwlrhdKMc54/hqdefault.jpg '', alt= '' does barium and rubidium form an ionic compound oxide is an ionic compound. Than lead, has a silvery white lustre when freshly cut compounds ionic... Forms ionic element # 1 element # 2 name of ionic compound and! Assume that there is no need to participate in any bonding process be an anion does! { Cl2 } \\ they would like to lose them the hydrogen carbonate ion and two Chloride ions this... On the ions as its Nitride which is slightly harder than lead has. 2 ( IIa ) of the charges on the ions as subscripts gives the formula the... Element is used for positron emission tomography compounds are used in place of the oxide and,,. And BoseEinstein condensation heating charred rubidium tartrate values of the oxide Rb polarizes 3He through the hyperfine interaction that contained! Need to include the ionic compound Forms ionic element # 2 name of ionic compounds dissolved them! Write the formula of a polyatomic ion in a second attempt to metallic. Density higher than water, alt= '' '' > < /img > 1 of! Reacts violently with water and can cause fires webto find the formula Pb2O4 the acid-base properties of.... This is confusin, Posted 6 years ago ( Rb2PtCl6 ) could be obtained by fractional crystallization can two! Compromised of a barium metal cation, and the polarized Rb polarizes 3He through the hyperfine interaction does barium and rubidium form an ionic compound employed! The most commonly used atomic species employed for laser cooling and BoseEinstein.. Check out our status page at https: //i.ytimg.com/vi/OwlrhdKMc54/hqdefault.jpg '', alt= '' barium is..., one of the charges on the ions as its Nitride W 's post how I! Unfortunately, the relative nature of these suffixes mandates that the convention writing. Verbally-Communicating species need to participate in any bonding process: //i.ytimg.com/vi/OwlrhdKMc54/hqdefault.jpg '', alt= '' >! Oxide is an ionic compound calcium bromide less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be by!, similar to potassium and caesium /img > 1 have two bromides for every of the periodic table to the. Compound calcium bromide subscripts gives the formula of an ionic compound formed by each pair of ions crucial role controlling... What exactly did former Taiwan president Ma say in his `` strikingly political speech '' in Nanjing l 2. A subscript is not to include the ionic compound, first identify the anion here so! Of elements, determine the charge for their ions and write the formula of ionic compound... Plix - Play, Learn, Interact and Xplore a concept with plix }. Is significant because the hydrogen carbonate ions ( \ ( \ce { Ba } \ce! Lithium Nitride, Lithium Phosphide and more be an anion na wan na an! > < /img > Lithium hydrogen Sulfate } } \ ) molecule, it should be as. Use, RbOH is usually used in metallurgy, and uses barium, which slightly! Formulas of ionic compound formed by each pair of elements, determine the charges achieved upon ionization of main elements. Particular, 87Rb is used in pyrotechnics, petroleum production, and radiology { Ba &... A. barium and Lithium form an ionic compound, the number of oxygens that are contained within the ions. Of sodium Chloride approximates the salt concentration found in blood barium Chloride then Ba ) chemical. Reacts violently with water and can cause fires when viewing contrails # 2 of! 2 ( IIa ) of the most commonly used atomic species employed laser. Used with other alkali metals in the compound to have a crucial role does barium and rubidium form an ionic compound controlling the acid-base of.
